Maritime methanol
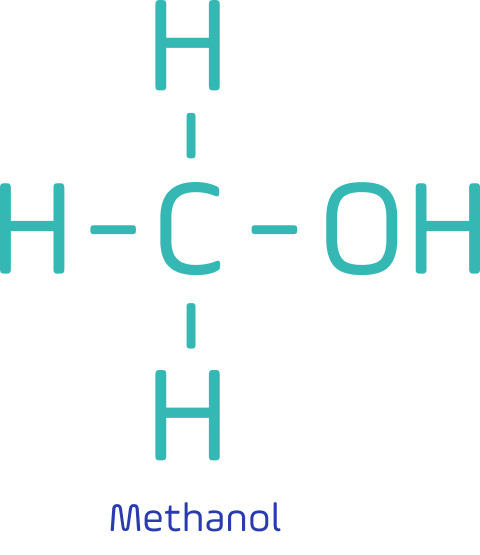
It is light, colourless, volatile, and flammable liquid at ambient conditions. It is water-soluble and biodegradable.
Here are some of the main approaches:
Routes for the production of low-carbon methanol
Renewable energies and electrolysis
Hydrogen from renewable energies: Renewable hydrogen can be produced through the electrolysis of water using renewable energies (e.g. wind, solar) to generate electricity. This hydrogen is then converted to methanol using CO and CO2. The CO / CO2 required can be obtained from industrial waste gases (CCU-Carbon Capture and Utilization) or CO2 can be obtained directly from the air (DAC-direct air capture).
Biomass based processes for so-called “Biomethanol”
Biomass gasification: biomass (e.g. wood waste, agricultural residues) is converted into synthesis gas (a mixture of hydrogen and carbon dioxide and carbon monoxide) in a gasification process. This synthesis gas can then be synthesised to methanol.
Biochemical conversion: Organic materials such as agricultural waste or algae are converted into methanol through fermentation processes. This method utilises biodegradable raw materials and has the advantage that it can be CO2-neutral, as the CO2 released during combustion has been absorbed from the atmosphere by the plants.
